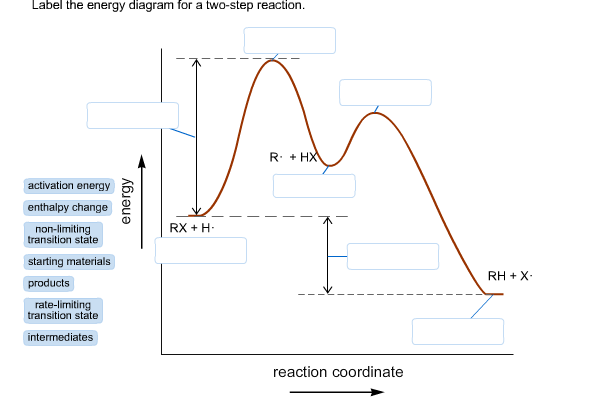
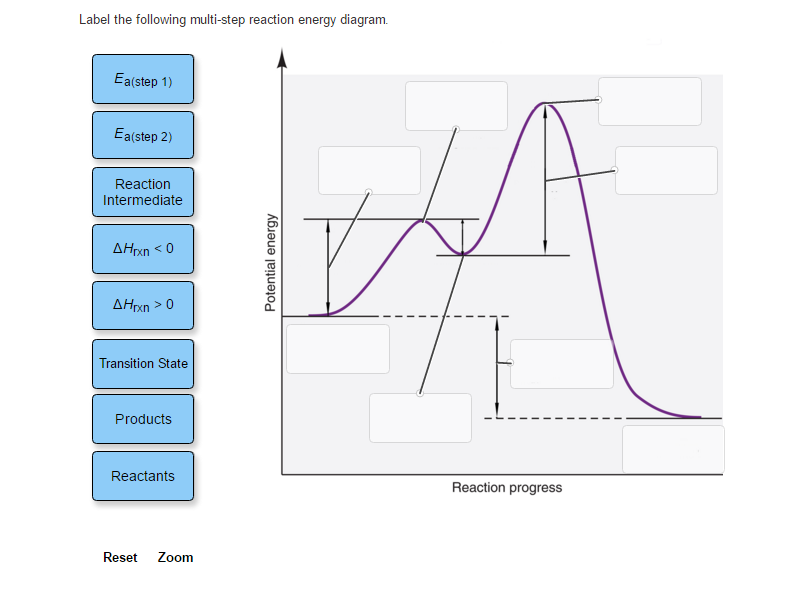
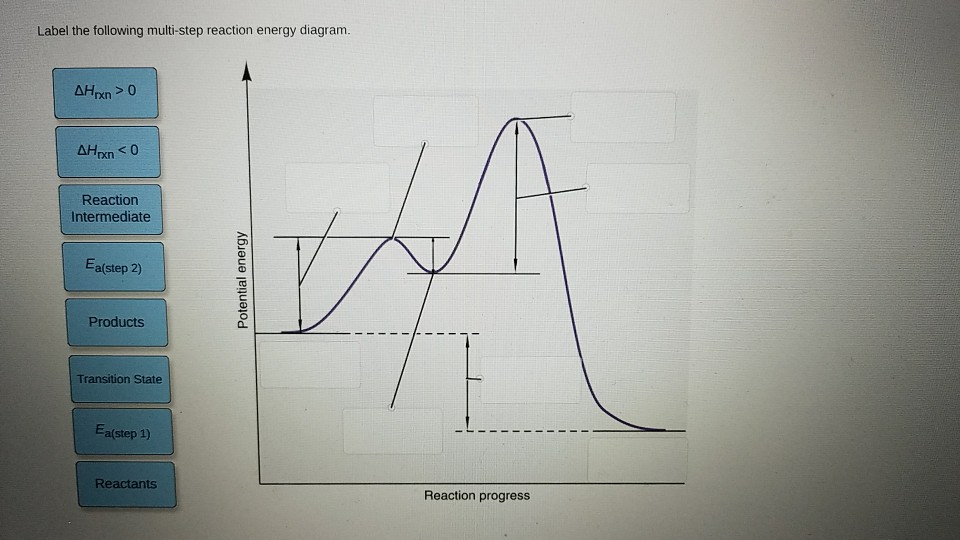
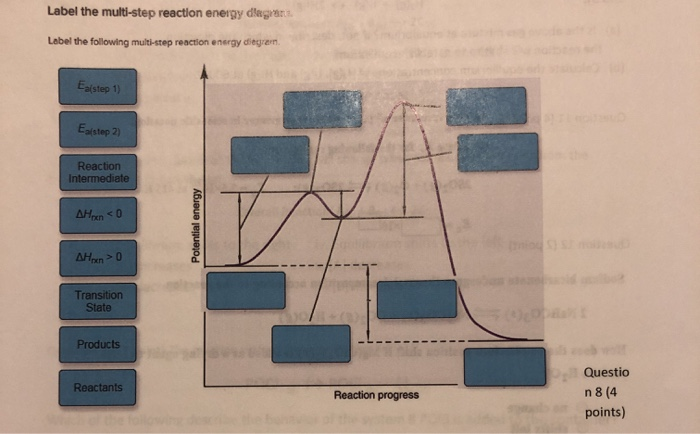
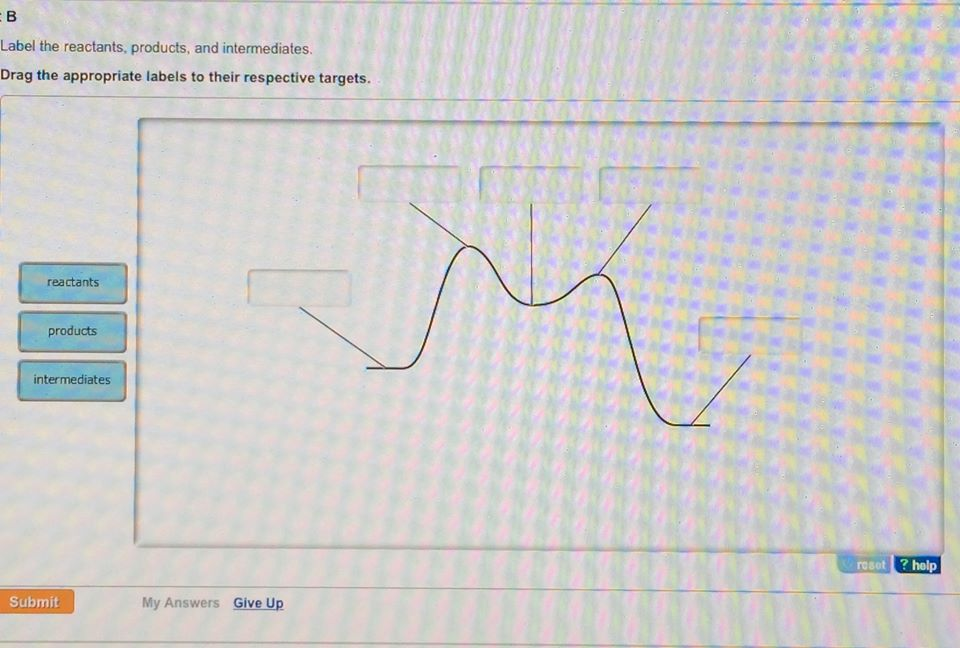
43 label the following multi-step reaction energy diagram
assignmentessays.comAssignment Essays - Best Custom Writing Services Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply. Multistep reaction energy profiles (video) - Khan Academy Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used to compare the activation energies of the different steps and identify the rate-determining step. The energy profile can also be used to determine the overall change in energy for the reaction.
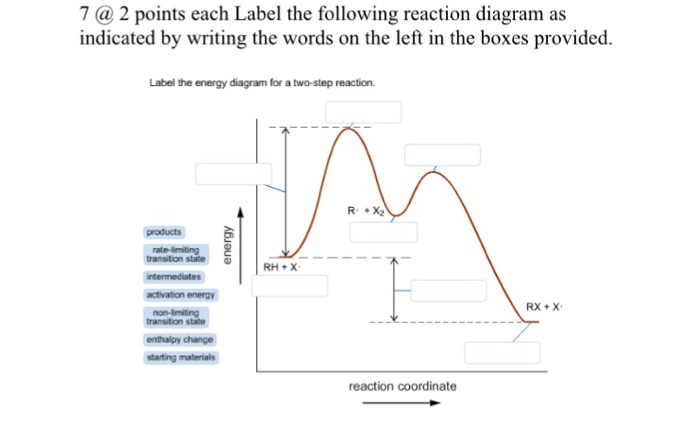
Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product.
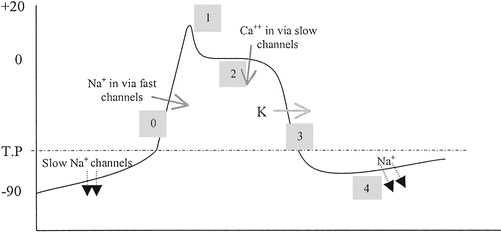
Label the following multi-step reaction energy diagram
How to Represent Electrons in an Energy Level Diagram So you put 8 electrons into your energy level diagram. You can represent electrons as arrows. If two electrons end up in the same orbital, one arrow faces up and the other faces down. The first electron goes into the 1s orbital, filling the lowest energy level first, and the second one spin pairs with the first one. achieverpapers.comAchiever Papers - We help students improve their academic ... We offer assignment help in more than 80 courses. We are also able to handle any complex paper in any course as we have employed professional writers who are specialized in different fields of study. From their experience, they are able to work on the most difficult assignments. The following are some of the course we offer assignment help in ... How to Draw Multi-Steps Energy Profile Diagrams - YouTube May 28, 2020 ... We'll go over how to label the reactants, products, transition state, activated complex, activation energy, ∆H delta H, forward reaction, ...
Label the following multi-step reaction energy diagram. Energy Diagram — Overview & Parts - Expii 1) Label the axes, the x-axis is labeled with reaction coordinate, and the y-axis is ... Finally, he shows you an energy diagram for multistep reactions. Energy Level Diagram - Different Energy Shells Around the Nucleus - BYJUS Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy levels is as follows: Chapter 14 B Flashcards | Quizlet The following mechanism has been proposed for the gas-phase reaction of H2 with ICl: H2 (g)+ICl (g)→HI (g)+HCl (g) HI (g)+ICl (g)→I2 (g)+HCl (g) Write rate laws for each elementary reaction in the mechanism. The slow step determines the rate law Rate = k [H2] [ICl] Unimolecular Reaction Rate Law A --> products = k [A] Recommended textbook solutions Rates, Temperature and Potential Energy Diagrams Worksheet Draw a potential energy (E p ) diagram for a reaction in which ∆H = 80 kJ/mol and E a = +28kJ/mol. Label the axes, activation energy, ∆H, site of the activated complex, reactants and products. 3. Using the potential energy diagrams for an endothermic and exothermic reaction shown, choose the letter that best fits each statement.
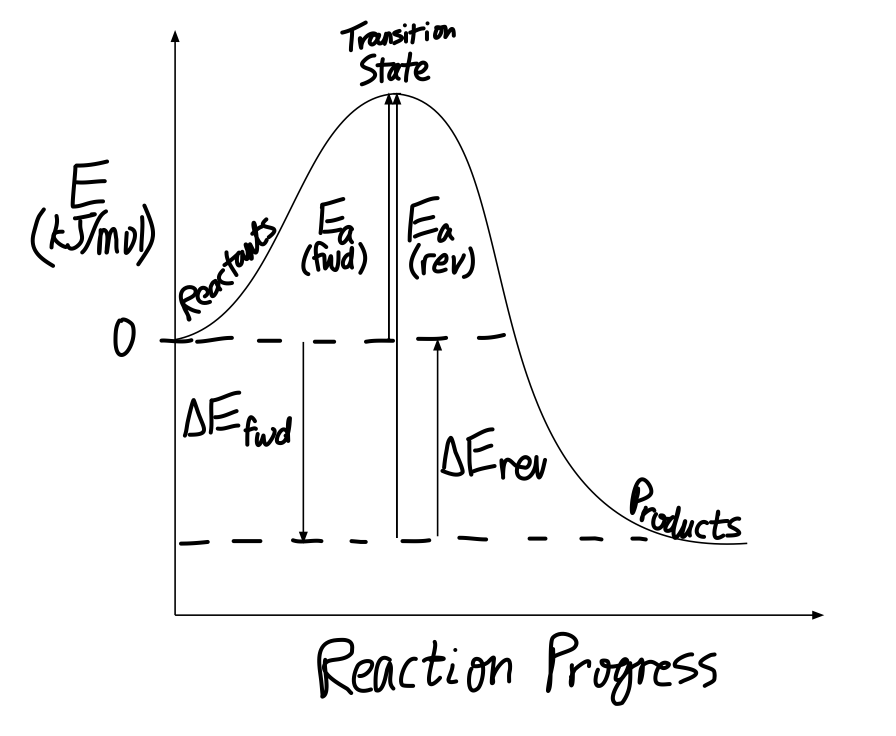
4.1 Energy and Metabolism - Concepts of Biology - 1st Canadian Edition A negative change in free energy also means that the products of the reaction have less free energy than the reactants, because they release some free energy during the reaction. Reactions that have a negative change in free energy and consequently release free energy are called exergonic reactions. Think: exergonic means energy is exiting the ... PDF 5.12 Spring 2003 Review Session: Exam #2 - MIT OpenCourseWare D. Reaction-Energy Diagrams 1. Thermodynamic Control 2. Kinetic Control 3. Hammond Postulate 4. Multi-Step Reactions 5. Chlorination of Methane E. Chlorination of Propane 1. Inequivalent Hydrogens (1°,2°,3°) 2. Relative Reactivity 3. Selectivity F. Bromination of Propane 1. Label the following multi-step reaction energy diagram. - Transtutors Label the following multi-step reaction diagram Label the following multi-step reaction energy diagram. Solved Label the following multi-step reaction energy | Chegg.com Question: Label the following multi-step reaction energy diagram. This problem has been solved! You'll get a detailed solution from a subject matter expert that ...
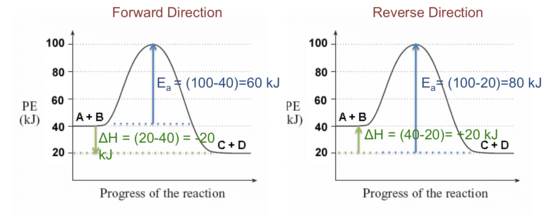
successessays.comSuccess Essays - Assisting students with assignments online Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply. Label the following multi-step reaction energy diagram. - OneClass Sep 22, 2020 ... Label the following multi-step reaction energy diagram. Answer. +20. Watch. 1. answer. 0. watching. 1,173. views. Energy Diagrams of Reactions | Fiveable To find the activation energy, you should be looking for two numbers: the potential energy of the reactants and the energy of the activated complex (the maximum point). (energy of activation complex) - (PEreactants) (100 kJ) - (40 kJ) = 60 kJ In other words, it takes 60 kJ of energy to complete the reaction. Reaction mechanism and rate law (article) | Khan Academy A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. The slowest step in a reaction mechanism is known as the rate-determining step.
Answered: (a) The diagram below shows the circuit… | bartleby Transcribed Image Text: (a) The diagram below shows the circuit used to measure the current-voltage (I-V) characteristic of an electrical component X. X On the diagram above, (ii) mark the position of the contact of the potentiometer that will produce a reading of zero on the voltmeter. Label this position P. Expert Solution
Metabolism Pathways-Examples, Diagrams, and Overview - Tutorsploit The catabolic pathway breaks down sugars and fats in food into carbon dioxide, water, and energy. An example of a catabolic pathway is the Metabolism of glucose to carbon dioxide and water, which occurs during cellular respiration. During this process, glucose is broken down and converted to energy.
Reaction Mechanisms and Multistep Reactions - Chemistry LibreTexts Sketch out an activation energy diagram for a multistep mechanism involving a rate-determining step, and relate this to the activation energy of the overall reaction. Write the rate law expression for a two-step mechanism in which the rate constants have significantly different magnitudes.
› 36548458 › Instrumentation_andInstrumentation & Control Process Control Fundamentals Differentiate feedback and feedforward control loops in terms of their operation, design, benefits, and limitations Perform the following functions for each type of standard process control loop (i.e., pressure, flow, level, and temperature): • State the type of control typically used and explain why it is used • Identify and describe ...
Mastering Biology Chapter 6 Flashcards | Quizlet glucose gas produced by photosynthesis O2 Drag the labels onto the equation to identify the inputs and outputs of cellular respiration. INPUTS--- 1 glucose (fuel) +6 O2 (gas we inhale) OUTPUTS--- 6CO2 (gas we exhale) + 6 H2O (water) + ATP (energy packets that cells use to do work
Cellular Respiration Equation, Types, Stages, Products & Diagrams As shown in the above diagram, glycolysis takes place in the cytosol. Glycolysis is referred to as a "ten enzyme-catalyzed reaction" but the overall simplified equation is: C6H12O6 + 2 NAD+ + 2 ADP + 2 P → 2 pyruvic acid, (CH3(C=O)COOH + 2 ATP + 2 NADH + 2 H+
ENERGY PROFILES FOR SIMPLE REACTIONS - chemguide This diagram shows that, overall, the reaction is exothermic. The products have a lower energy than the reactants, and so energy is released when the reaction happens. It also shows that the molecules have to possess enough energy (called activation energy) to get the reactants over what we think of as the "activation energy barrier".
PDF time (s) conc. (M) 0 1.284 1.4 1.135 2.8 0.986 4.2 0.837 7.0 0 - gccaz.edu 6. (6.5 points) Assume a certain chemical reaction releases 57kJ/mol of heat. Use the following diagram to show the energy change during the reaction. Make sure to label: products, E a, ∆H (change in heat energy). Extra Credit (2 points): What is the chemical species at the highest point in the energy curve called? Label it on the diagram.
PDF Rates, Temperature and Potential Energy Diagrams Worksheet a) Draw a potential energy diagram for this reversible reaction. Your starting value for the reactants might be different that is ok, as long as you show the proper E a and ∆H values. b) Calculate the enthalpy change (∆H) for each reaction. i) ΔH forward = 137 kJ/mol i) ΔH reverse = 137 kJ/mol 6.
18.5 Multi step Reaction - CK-12 Feb 23, 2012 ... Draw the potential energy diagram for the following multi-step reaction \begin{align*}(\triangle H < 0)\end{align*}. Properly label the ...
Potential Energy Diagrams | Chemistry for Non-Majors | | Course Hero A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. This ...
pubs.acs.org › toc › esthagEnvironmental Science & Technology | Ahead of Print Articles ASAP (as soon as publishable) are posted online and available to view immediately after technical editing, formatting for publication, and author proofing.
Exothermic and endothermic reactions - AQA - BBC Bitesize An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
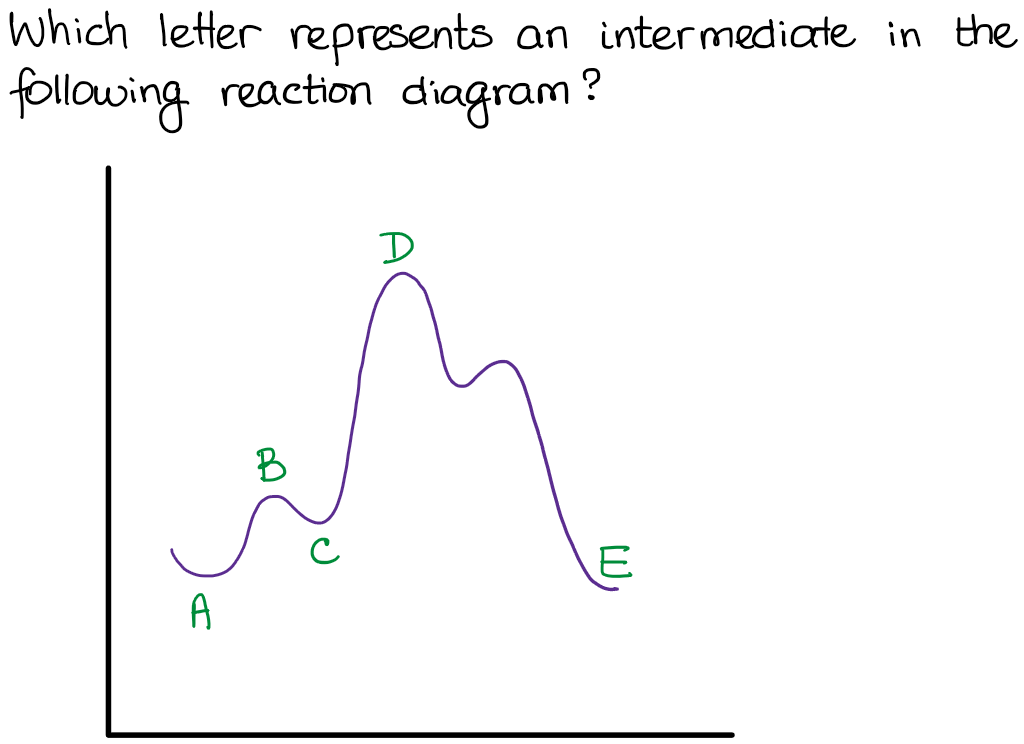
Answered: Reaction progress Potential Energy | bartleby Q: The diagram below shows the energy profile for a reaction. D В E C A Reaction progress Species… D В E C A Reaction progress Species… A: transition state is a very short lived state of atoms ,in the transition state , Energy of atoms…
Cellular Respiration Diagram - Biology Wise The energy released is in the form of ATP molecules that are used to carry out various functions of the cell. The cellular respiration equation is as follows: C6H12O6 + O2 ――> H2O + CO2 + 36ATP. The following diagram of cellular respiration will give a better understanding of this process.
Energy level diagrams - Why are there energy changes in chemical ... Energy level diagrams are used to model energy changes during reactions. They show the relative energy levels of the products and reactants. Exothermic reaction The energy level decreases in an...
Chemistry.doc - For each molecule, circle and name the... Label them on the diagram. ... An intermediate on the other hand is a transient product that is represented by the lowest energy minimum in a multi-step reaction. ... Problem 6.9 Predict the products of the following polar reaction, a step in the citric acid cycle for food metabolism, ...
Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1
coursehelponline.comCourse Help Online - Have your academic paper written by a ... We offer assignment help in more than 80 courses. We are also able to handle any complex paper in any course as we have employed professional writers who are specialized in different fields of study. From their experience, they are able to work on the most difficult assignments. The following are some of the course we offer assignment help in ...
Reaction Mechanisms - Chem1 The gas-phase formation of HI from its elements was long thought to be a simple bimolecular combination of H 2 and I 2, but it was later found that under certain conditions, it followes a more complicated rate law. 2 Multi-step (consecutive) reactions Mechanisms in which one elementary step is followed by another are very common. step 1: A + B → Q
› questions-and-answers › learningAnswered: Learning Task 1 Give an example of a… | bartleby Q: multi-step reaction OH multi-step reaction HO B A: Click to see the answer Q: The following diagrams represent a hypothetical reaction A ⇋ B, with A represented by white spheres…
Solved Label the following multi-step reaction energy | Chegg.com Question: Label the following multi-step reaction energy diagram. Eatstep 1) Transition State Transition State Eastep 2) Eastep 1) Ea (step 2) Transition State Reactants Potential energy Products Reactants Reaction Intermediate ΔΗκη <0 AH 0 Reaction Intermediate Products ΔΗρκιν >0 Reaction progress This problem has been solved! See the answer
Energy Diagram for a Two-Step Reaction Mechanism Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and
How to Draw Multi-Steps Energy Profile Diagrams - YouTube May 28, 2020 ... We'll go over how to label the reactants, products, transition state, activated complex, activation energy, ∆H delta H, forward reaction, ...
achieverpapers.comAchiever Papers - We help students improve their academic ... We offer assignment help in more than 80 courses. We are also able to handle any complex paper in any course as we have employed professional writers who are specialized in different fields of study. From their experience, they are able to work on the most difficult assignments. The following are some of the course we offer assignment help in ...
How to Represent Electrons in an Energy Level Diagram So you put 8 electrons into your energy level diagram. You can represent electrons as arrows. If two electrons end up in the same orbital, one arrow faces up and the other faces down. The first electron goes into the 1s orbital, filling the lowest energy level first, and the second one spin pairs with the first one.

:max_bytes(150000):strip_icc()/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)

























Komentar
Posting Komentar