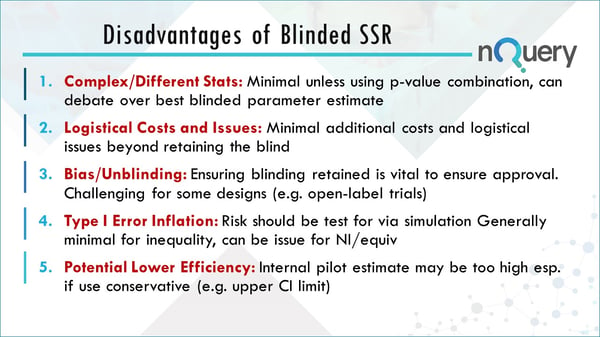
38 open-label study disadvantages
UpToDate Sep 27, 2022 · A recent study evaluated 2190 students (mean age 13.3 years) who had participated in a prior randomized trial that compared various preventive strategies (universal web-based prevention, selective prevention for high-risk students, or a combination of the two) with health education as usual . At seven-year follow-up, individuals from all three ... Reducing bias in open-label trials where blinded outcome assessment is ... This can be an issue in trials assessing surgical interventions, device trials, or other non-pharmacologic interventions, which are more difficult to blind than traditional drug trials [ 4 ]. Many such trials are therefore open-label, where patients, clinicians, and care providers are aware of treatment allocations.
(PDF) What is an open label trial? - ResearchGate The small sample size and the dropout rate of 40% limit the generalisability of the results, as well as increase the risk of type 2 errors (Sullivan & Feinn, 2012). Another limitation is linked to...
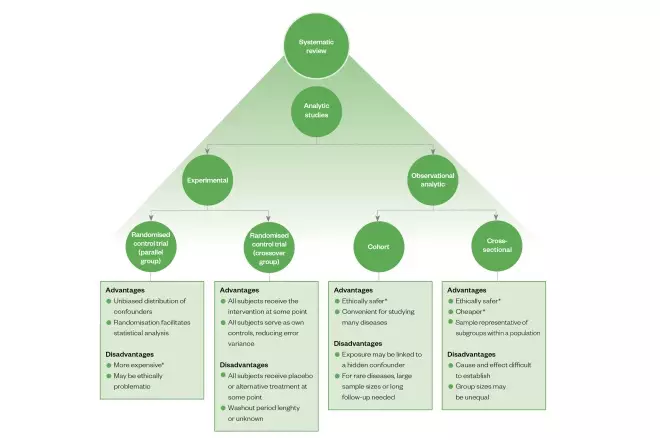
Open-label study disadvantages
Gastrointestinal injury associated with NSAID use: a case ... Jan 22, 2015 · Prevalence of nonsteroidal anti-inflammatory drug use. Adequate pain management is a widespread clinical concern, and both prescription and over-the-counter (OTC) nonsteroidal anti-inflammatory drugs (NSAIDs) are frequently used for pain relief.1 NSAID use may also be increasing, as indicated by a 2010 National Health Interview Survey (NHIS) that found that 12.8% of adults in the United States ... Open-Label Extension Studies | SpringerLink The ethical concern that informed consent cannot be given is clear: response while receiving placebo or lack of response to the new medicine by the patient in the 'active' arm of the phase III study would not provide a sensible basis for participation in an open-label extension study. Open-label study | definition of open-label study by Medical dictionary open-label study: a study in which there is no blinding of treatments.
Open-label study disadvantages. Bias for Patient-Reported Outcomes in Open-Label Cancer Trials: How Big ... A common concern with patient-reported outcomes (PROs) in open-label trials is that a patient's knowledge of treatment received could influence their view and reporting of their symptoms. With this in mind, members of the US Food and Drug Administration explored the possibility of such bias in a recent viewpoint published in JAMA Oncology. What is an open label trial? | The BMJ In an open trial, ascertainment bias can also occur on behalf of the participants. Participants know their treatment allocation and, for example, might be disappointed if not allocated their preferred treatment, with the result that they report worse scores for the outcome measures than were experienced. 14 Advantages and Disadvantages of a Randomized Controlled Trial - Vittana List of the Advantages of Randomized Controlled Trials. 1. Randomization prevents the deliberate manipulation of results. A randomized controlled trial works to prevent skewing or the deliberate manipulation of results by researchers or participants. Because each subject gets assigned to a specific group randomly, the removal of choice works to ... Medication Abortion Up to 70 Days of Gestation | ACOG A study from China found that patients who had a prior mifepristone abortion had lower odds of preterm birth compared with those who had never been pregnant (adjusted OR, 0.77; 95% CI, 0.61–0.98), and the frequencies of low-birth-weight infants and mean lengths of pregnancy were similar in both groups 127. No significant differences were ...
Oral versus Intravenous Antibiotics for Bone and Joint ... Jan 31, 2019 · We recruited 1054 participants (including 228 from the single-center internal pilot study) across 26 U.K. sites (median, 8 participants per site; interquartile range, 4 to 24) between June 2010 ... Pilot Studies: Common Uses and Misuses - NCCIH Rather than focusing on feasibility and acceptability, too often, proposed pilot studies focus on inappropriate outcomes, such as determining "preliminary efficacy." The most common misuses of pilot studies include: Attempting to assess safety/tolerability of a treatment, Seeking to provide a preliminary test of the research hypothesis, and Open-Label Trial - an overview | ScienceDirect Topics Open-label studies lack the rigor of blinded studies. Since the lack of blinding can introduce significant bias, reserve the use of open-label studies for situations in which blinding is neither feasible nor ethical or in cases where the outcome is completely objective, such as survival. Some situations include: • Open label extension studies: research or marketing? - PMC Summary points. Open label extension studies are a common adjunct to double blind randomised controlled trials of new drugs. The aim of open label extension studies often seems to be to enable continued use of a new drug for marketing or compassionate purposes rather than to increase knowledge. The continued use of a new drug on compassionate ...
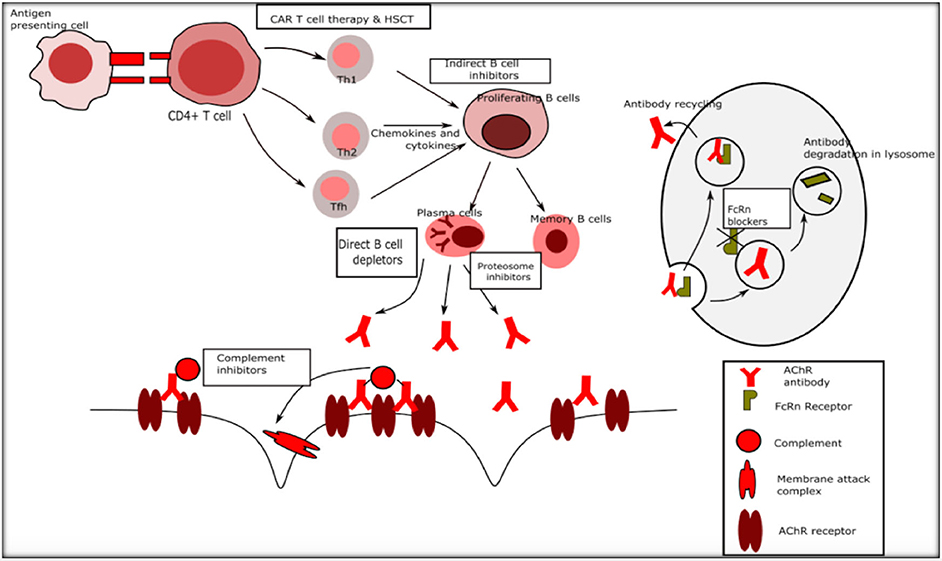
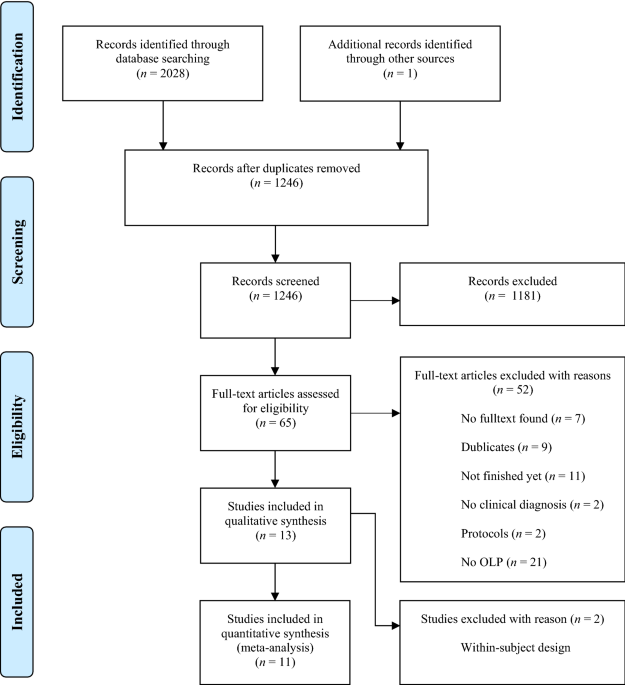
National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine. PDF ACKNOWLEDGMENTS - National Pharmaceutical Council In practice, each research approach has advantages and disadvantages, and the research approach for a CER question should be selected based upon the specific features or characteristics of the study question. The purpose of this document is to provide brief descriptions of both experimental and nonexperimental study designs and methods that may The Advantages & Disadvantages of Unblinded Sample Size Re ... - Statsols The advantages of unblinded sample size re-estimation clinical trials. Earlier Decisions. You inherit the Group Sequential Design which allows the researcher to stop early for efficacy or futility. This allows the ability to adjust the trial to the reality of what the effect size is telling you will happen in the trial. Reduced Potential Cost. Effects of open-label placebos in clinical trials: a ... - Nature These trials assessed effects of OLPs on back pain, cancer-related fatigue, attention deficit hyperactivity disorder, allergic rhinitis, major depression, irritable bowel syndrome and menopausal...
Glutathione Uses, Benefits & Dosage - Drugs.com Herbal Database Jul 14, 2022 · An open-label pilot study (N=34) evaluated effects of oral glutathione 300 mg/day for 4 months in patients with nonalcoholic fatty liver disease.Honda 2017 Serum ALT levels were significantly decreased from baseline, as were levels of triglycerides and ferritin. Researchers also noted that diabetic status and age were correlated with ALT response.
Cardiovascular clinical trials in Japan and controversies ... - Nature Table 1 Advantages and disadvantages of the PROBE design compared with double-blinded design. ... (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study.
Understanding Clinical Trial Terminology: What is an Open Label ... Often, clinical trials use a double-blind approach: study participants and researchers don't know which treatment the patient is receiving. Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving.
Investigating Potential Bias in Patient-Reported Outcomes in Open-label ... At the same time, randomized oncology trials are increasingly open-label, and the accelerated approval pathway is frequently sought for oncology products based on single-arm trials. 7 Rigorous characterization of symptoms and function using PRO measures can complement current trial outcomes, and this information may be incorporated into ...
PDF Blinding Sponsors for Open Label Studies: Challenges and Solutions - MWSUG the study is ongoing. The practice helps increase credibility of study results and thus should be followed, particularly for registration studies. In open label studies, in addition to treatment assignment data itself, many other data elements in the CRF data can reveal treatment assignment. Those data elements include (but not limited to): •
Open-label extension studies: do they provide meaningful ... - PubMed Negative aspects of open-label extension studies revolve around their use as a marketing tool, as they build a market for the drug and generate pressure for subsidised access to the drug from consumers and their physicians.
Epidemiology and Clinical Research Design, Part 1: Study Types. It is difficult to ensure that the exposed and control groups have the same risk of outcome (other than the exposure). In addition to known baseline characteristics for which the exposed and control groups are matched, there may be unknown prognostic factors that are unevenly distributed in the 2 groups contributing to the outcome.
Effectiveness studies: advantages and disadvantages - PMC Go to: - Cannot make as meaningful clinical comparisons between agents Highly restricted inclusion criteria to reduce confounding biases Randomization and blinding, also to reduce bias - Fewer treatment adjustments are allowed - Higher internal validity for clinical effects - Higher internal validity for adverse effects, tolerability
External and internal validity of open label or double-blind trials in ... In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-randomized treatment decisions and on reporting of outcomes. However, a blinded trial is not always feasible.
External and internal validity of open label or double-blind trials in ... disadvantages of open-label or double-blind trials is currently underway and interpretation oftrialresults isoften focusedon this matter. In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-random-
Open-Label Trial | NIH - HIV.gov In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Skip to main content Get the latest public health information from CDC ... Double-Blind Study. Print. Print this term. Download Glossary. English Version PDF(3.13MB) Spanish Version PDF(3.16MB) CONNECT WITH US ...
Case report - Wikipedia In medicine, a case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient.Case reports may contain a demographic profile of the patient, but usually describe an unusual or novel occurrence.
Analysis of clinical trials - Wikipedia If a patient drops out of the study after the third week, then this value is "carried forward" and assumed to be his or her score for the 5 missing data points. The assumption is that the patients improve gradually from the start of the study until the end, so that carrying forward an intermediate value is a conservative estimate of how well ...
What is an Open-Label Clinical Trial? - News-Medical.net Day, R.O & Williams, K.M (2007) Open-label extension studies: do they provide meaningful information on the safety of new drugs? Drug Saf. 30(2) pp. 93-105 [online] Accessed online 16 th March 2022.
Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized.
External and internal validity of open label or double ... - ResearchGate Open-label study designs often lack randomization or blinding which can result in detection, reporting, and response biases (Beyer-Westendorf and Büller, 2011; Hróbjartsson et al., 2014 ...
Open-label study | definition of open-label study by Medical dictionary open-label study: a study in which there is no blinding of treatments.
Open-Label Extension Studies | SpringerLink The ethical concern that informed consent cannot be given is clear: response while receiving placebo or lack of response to the new medicine by the patient in the 'active' arm of the phase III study would not provide a sensible basis for participation in an open-label extension study.
Gastrointestinal injury associated with NSAID use: a case ... Jan 22, 2015 · Prevalence of nonsteroidal anti-inflammatory drug use. Adequate pain management is a widespread clinical concern, and both prescription and over-the-counter (OTC) nonsteroidal anti-inflammatory drugs (NSAIDs) are frequently used for pain relief.1 NSAID use may also be increasing, as indicated by a 2010 National Health Interview Survey (NHIS) that found that 12.8% of adults in the United States ...











![PDF] The Clinical Viewpoint: Definitions, Limitations of ...](https://d3i71xaburhd42.cloudfront.net/0d341492d445073e64d1ccb6985e4ad763a3d1f7/2-Table1-1.png)



![PDF] Long-acting, injectable antiretroviral therapy for the ...](https://d3i71xaburhd42.cloudfront.net/274c51f88b6396ab37b2f6c6c3b31b9d302c7643/2-Table2-1.png)



Komentar
Posting Komentar