38 draw and label ph scale
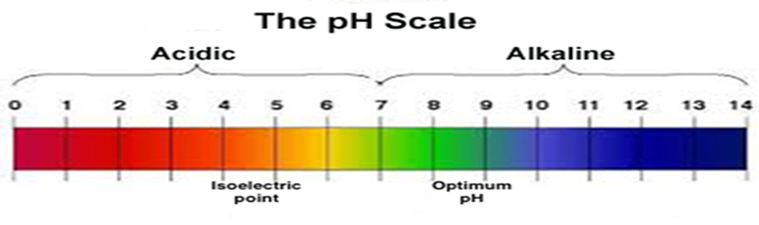
pH Scale - Acids and Bases A pH of 7 is neutral on the scale, greater than 7 is a base and less than 7 is an acid. Strong acids are mostly ranged at a pH of 0-2, strong bases have a range at a pH of 12-14. Colour Indicators Colour Indicators are used to determine how acidic, basic or neutral the solution is. PH Scale in Simple Terms - YouTube What is the pH scale? The letters pH stand for the potential of hydrogen and is a measure of the concentration of hydrogen ions in a water-based substance?pH...
2. Draw and label the \( \mathrm{pH} \) scale. 3. | Chegg.com 2. Draw and label the \ ( \mathrm {pH} \) scale. 3. Define the terms below: a. Provide the chemical reaction for the dissociation of water. b. Acid c. Base d. Neutral c. Buffer Coqright \ ( 62 \mathrm {Dl} 9 \) hy Tonna Harris Haller. Trxas ARM Univerity Why does it matter? The understanding of pH is essential to life and its processes.
Draw and label ph scale
PDF Lesson 8: Acids, Bases, and the pH Scale I. Time II. Materials Handout: a. Have students draw and label the pH scale without looking at their notes. b. Give examples of biological buffers and ask the students to explain how they work. c. Student completion of "Acids, Bases, and the pH Scale" handouts will assess the above objectives. VI. Related Links/Resources: a. The EU Mission for the Support of Palestinian Police and Rule ... Oct 14, 2022 · EUPOL COPPS (the EU Coordinating Office for Palestinian Police Support), mainly through these two sections, assists the Palestinian Authority in building its institutions, for a future Palestinian state, focused on security and justice sector reforms. This is effected under Palestinian ownership and in accordance with the best European and international standards. Ultimately the Mission’s ... Draw a pH scale and label water, hydrochloric acid, and sodi - Quizlet Find step-by-step Biology solutions and your answer to the following textbook question: Draw a pH scale and label water, hydrochloric acid, and sodium hydroxide in their general areas on the scale.. ... refer to answer to see the drawing of pH scale. \textbf{\color{#4257b2}Please refer to answer to see the drawing of pH scale.} Please refer to ...
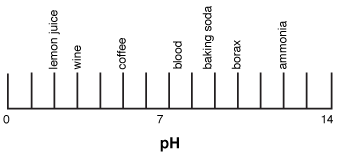
Draw and label ph scale. Yahoo News - Latest News & Headlines The latest news and headlines from Yahoo! News. Get breaking news stories and in-depth coverage with videos and photos. Solved Draw a pH scale and add to it the substances | Chegg.com Draw a pH scale and add to it the substances listed below. Do not forget to label the pH scale correctly Lemon juice is more acidic then orange juice, who is also more acidic then blood. Whereas toothpaste is less basic then oven cleaners. Both substances are more basic then baking soda. The pH scale - Acids, bases and salts - (CCEA) - BBC Bitesize The pH scale measures a solution's acidity or alkalinity. The range for the pH scale is 0 (strong acid) to 14 (strong alkali). pH 0 - 2: strong acid pH 3 - 6: weak acid pH 7: neutral pH 8 ... pH, pOH, and the pH scale (article) | Khan Academy Let's go through the calculation step-by-step. Step 1. Calculate the molar concentration of. Molar concentration is equal to moles of solute per liter of solution: To calculate the molar concentration of , we can use the known values for the moles of and the volume of solution: The concentration of in the solution is .
pH Scale - PhET pH Scale - PhET Draw neat and labeled diagram of pH scale? - Toppr Ask Solution Verified by Toppr The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0 Similar questions > Acids, Bases, and the pH Scale (Interactive Tutorial) Acidic and basic solutions are measured on the pH (pronounced "P" "H") scale. On the pH scale, the acids with the most hydrogen ions are at 0 (or rarely, below 0). The most concentrated bases are at pH 14 (or above). A neutral liquid is 7. The scale works by powers of 10, so a solution with pH 5 is 10 times more acidic than a solution that is pH 6. How do you draw the pH scale? - Answers The pH scale is often indicated as a vertical bar graph with scaled numbers from 0 to 14 (top to bottom). The lower numbers at the top are the more acidic pH, while the higher numbers near the...
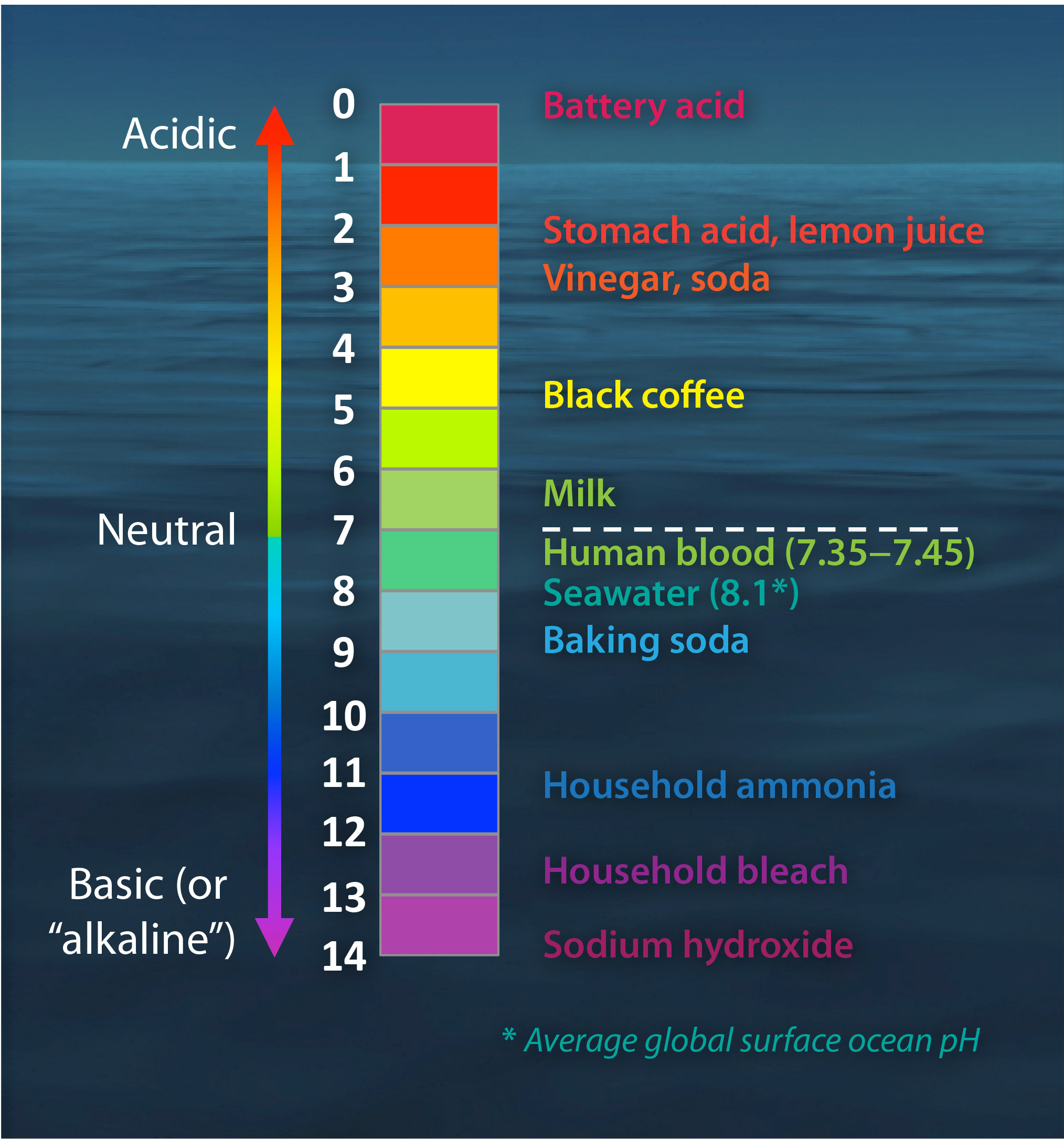
The pH scale - BBC Bitesize The pH scale is a number scale from 0 to 14. It tells us how acidic or alkaline an aqueous solution is. The pH scale is used to classify solutions as acidic, alkaline or neutral. Neutral solutions... Acids, Alkalis, and the pH Scale - Compound Interest Human blood has a pH value that's always slightly alkaline, between 7.35-7.45. If we were able to purposefully change the pH of blood outside of this small range, we could actually cause ourselves a good deal of harm; even a pH change of 0.5 either way could result in irreversible cell damage. pH Scale | U.S. Geological Survey The range goes from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water. Water that has more free hydrogen ions is acidic, whereas water that has more free hydroxyl ions is basic. バンスクリップの通販ショップ | 激安アクセサリー通販... アクセサリー通販lupis(ルピス)では人気のバンスクリップを販売しています。新商品が毎日入荷!お得な割引クーポンも ...
Flatiron Research Fellow, CCA in New York, NY for Simons ... The CCA's mission is to create, develop, and disseminate computational methods, tools, and frameworks that allow scientists to build or analyze big astronomical datasets, and to use computational and statistical techniques to understand complex, multi-scale physics in astrophysical systems ranging in scales from planets to the Universe.
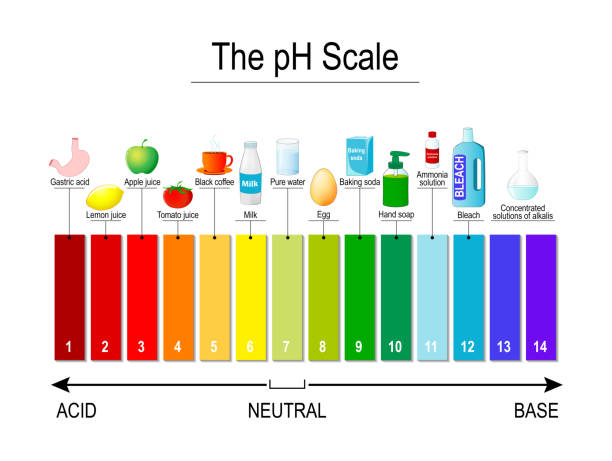
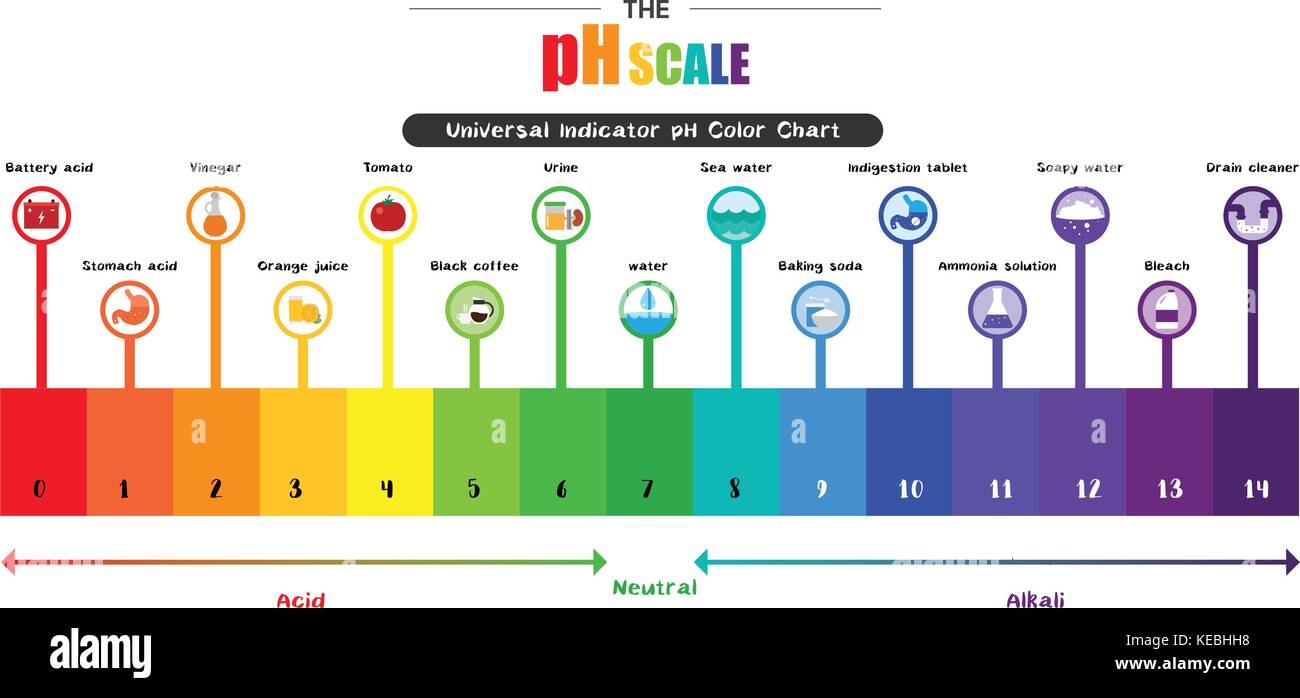
Acid_Base Project - Tyrone & Noreania (1).pptx - Acid According to an article called "pH and water", it states that "pH is a measure of how basic/acidic a substance is (or water).The range goes from 0-14, with 7 being neutral". This proves what pH is by directlyexplaining what it measures and its scale. 2. Provide a colored pH scale with indications of both strong and weak acids and bases.
The Hollywood Reporter The Definitive Voice of Entertainment News Subscribe for full access to The Hollywood Reporter. See My Options Sign Up
The pH Scale | Biology for Majors I | | Course Hero The pH scale is, as previously mentioned, an inverse logarithm and ranges from 0 to 14 (Figure 1). Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. Extremes in pH in either direction from 7.0 are usually considered inhospitable to life.
pH Scale: Acids, bases, pH and buffers (article) | Khan Academy The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although it's possible to get a pH below 0 or above 14. Anything below 7.0 is acidic, and anything above 7.0 is alkaline, or basic. Image modified from " Water: Figure 7 ," by OpenStax College, Biology, CC BY 4.0. Modification of work by Edward Stevens.
3. Draw a pH scale and label water, hydrochloric acid, and sodium ... The pH scale has been drawn below. Explanation: pH sacle may be defined as the scale that determines the pH of acids and bases. 0 represent the neutral compounds. The compounds that pH less than 7 acts as acid whereas the compounds that have pH greater than 7 acts as base.
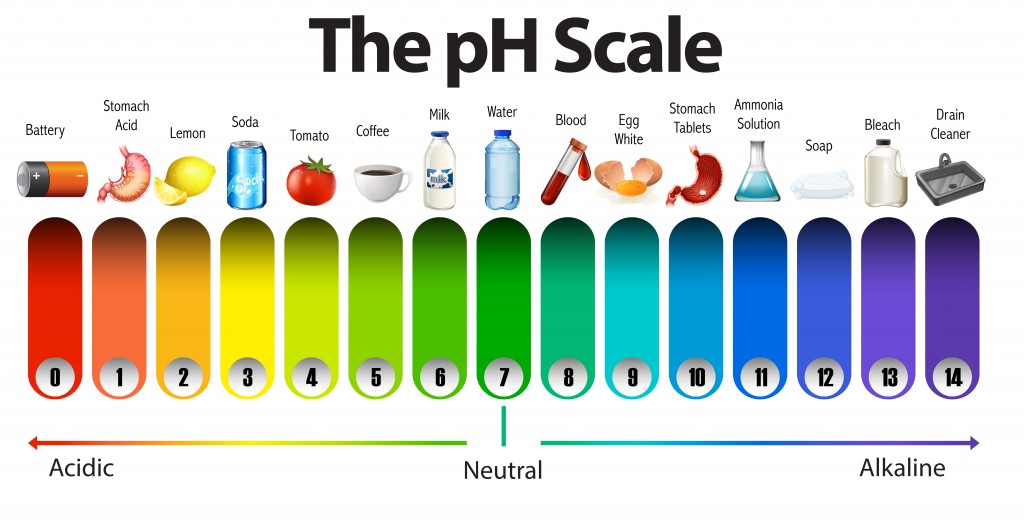
The pH scale with some common examples - NOAA Pacific Marine ... The pH scale, with examples of common solutions and their pH values. Download/View. For commercial use please contact us.
Diagram of the pH scale with examples of acidic, neutral and alkaline ... Download this stock image: Diagram of the pH scale with examples of acidic, neutral and alkaline substances. - G156N5 from Alamy's library of millions of high resolution stock photos, illustrations and vectors. ... acid acidic acidity alkaline annotated art artwork battery bleach chemistry diagram drain cleaner drawing graphic illustration info ...
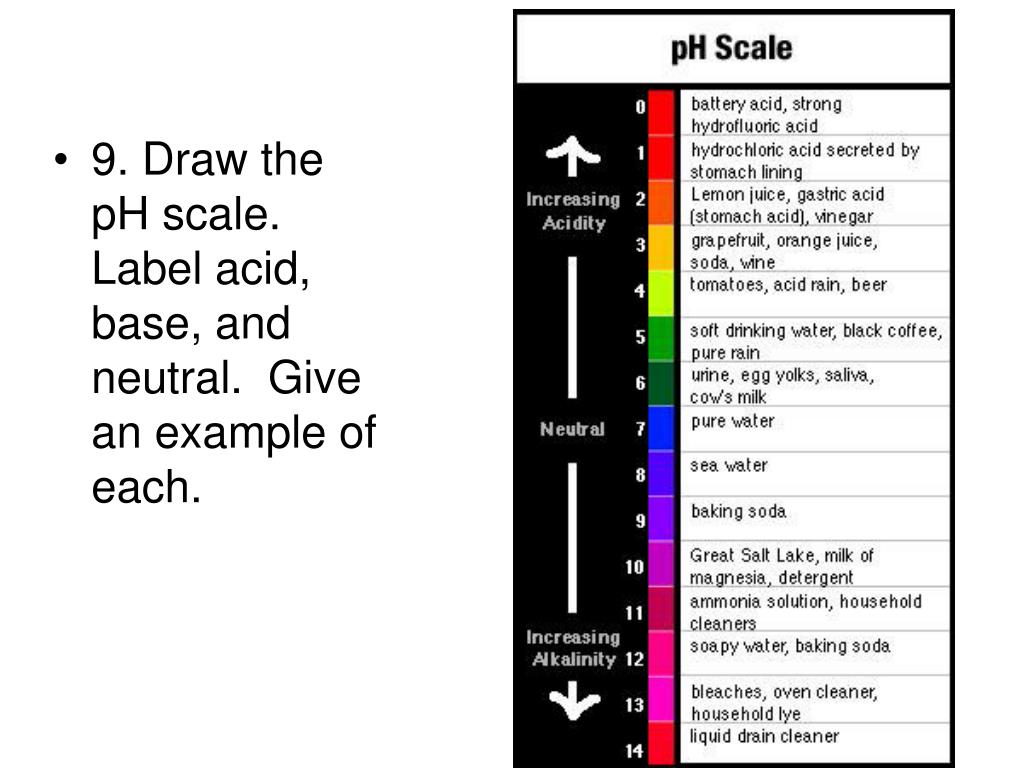
PDF pH Scale Activity - birdvilleschools.net 1. On the construction paper, NEATLY draw a pH scale. 2. Scale the line from 0 to 14 with a mark for each number. 3. ... Strong Base Weak Base Neutral . 4. Color & Label the pH on the picture. Cut out & paste in the correct sections of the pH scale. Window cleaner pH = stomach acid pH = lemon juice pH = vinegar pH = orange juice pH =
Interactive Notebook: Acids and Bases pH Scale - Teachers Pay Teachers Students learn about the pH scale by discovering common examples of substances with different pH values. Students use the Internet to search substances with specific pH values, then draw and label those substances in the worksheet. Students then color the given pH scale reflected in pH indicator pap...
pH Of Acids And Bases - BYJUS Example. Thus, the pH of an acidic solution of HNO 3 (10 -3 M) = 3, a basic solution of KOH having [OH -] =10 -4 M and [H 3 O +] =10 -10 M will have a pH = 10. pH of acids is generally less than 7 whereas for bases it is greater than 7. At 298 K, ionic product of water, K w can be given as:. K w = [H 3 O +] [OH -] = 10 -14. Taking the negative logarithm of RHS and LHS, we deduce
Assignment Essays - Best Custom Writing Services Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
pH Scale | U.S. Geological Survey The scale has values ranging from zero (the most acidic) to 14 (the most basic). As you can see from the pH scale above, pure water has a pH value of 7. This value is considered neutral—neither acidic or basic. Normal, clean rain has a pH value of between 5.0 and 5.5, which is slightly acidic.
Course Help Online - Have your academic paper written by a ... You can get help on any level of study from high school, certificate, diploma, degree, masters, and Ph.D. some of the subject areas we offer assignment help are as follows: Art Architecture
pH Scale Flashcards | Quizlet pH. abbreviation meaning "potential for hydrogen." pH Scale. a scale used to define the levels of hydrogen (H) or hydroxide (OH) ions in a solution. Ranges from 0 (acidic) to 14 (basic/alkaline), with 7 being neutral. Hydrogen Ion. an ion of hydrogen (H) created by the breaking up of a water molecule during the mixing of a solution.
Draw a pH scale and label water hydrochloric acid and sodium hydroxide ... Draw a pH scale and label water hydrochloric acid and sodium hydroxide in their general areas on the scale Advertisement Silvarziyankoh is waiting for your help. Add your answer and earn points. peterdaly See attached scan for answer which is pdf file Scan 0009 or 0.01M HCl is pH of 2, water is pH of 7 and NaOH concentrated is pH of 14.
PhET PhET
Draw a pH scale and label water, hydrochloric acid, and sodi - Quizlet Find step-by-step Biology solutions and your answer to the following textbook question: Draw a pH scale and label water, hydrochloric acid, and sodium hydroxide in their general areas on the scale.. ... refer to answer to see the drawing of pH scale. \textbf{\color{#4257b2}Please refer to answer to see the drawing of pH scale.} Please refer to ...
The EU Mission for the Support of Palestinian Police and Rule ... Oct 14, 2022 · EUPOL COPPS (the EU Coordinating Office for Palestinian Police Support), mainly through these two sections, assists the Palestinian Authority in building its institutions, for a future Palestinian state, focused on security and justice sector reforms. This is effected under Palestinian ownership and in accordance with the best European and international standards. Ultimately the Mission’s ...
PDF Lesson 8: Acids, Bases, and the pH Scale I. Time II. Materials Handout: a. Have students draw and label the pH scale without looking at their notes. b. Give examples of biological buffers and ask the students to explain how they work. c. Student completion of "Acids, Bases, and the pH Scale" handouts will assess the above objectives. VI. Related Links/Resources: a.








:max_bytes(150000):strip_icc()/definition-of-ph-in-chemistry-604605_final-5c8fac8446e0fb00017700d1.png)












Komentar
Posting Komentar